LIU Xiaoli1 LIU Yang1 Zhang WEI1 JIANG Yongmei1
GU Chunju1 ZHU Meifang1** Adler H. J.2
1. State Key Lab for Modification of Polymer Materials and Chemical Fibers College of Material Sci. & Eng., Donghua University, Shanghai 200051, China
2. Institute of Macromolecular Chemistry and Textile Chemistry, Dresden University of Technology, Mommsenstr. 13, D-01069 Dresden, Germany
Polymer hydrogels as a kind of soft materials have attracted increasing scientific and technological interest in past several decades. These materials have been applied widely in many fields [1], such as molecular filter , superabsorbent , and contact lenses etc. However,synthetic polymeric hydrogels are seldom used as mechanical devices due to the lack of mechanical strength. Therefore, it is very imperative to improve their mechanical properties for wider use of polymeric hydrogels.
Recently, three novel hydrogels with good mechanical properties have been reported: a‘topological gel (TP gel)’ using figure-of-eight as crosslinkers , a ‘double network gel (NC gel)’ by IPN methods and ‘nanocomposite gel (NC gel)’ using clay as crosslinker .
Compared with other two gels, NC gel has an important advantage: the simplicity of synthesis. It is easy to be prepared by in situ polymerization and to induce new structure. In past several years,some properties, structure and forming mechanism of NC gels have also been studied by different methods [10~14]. Most of the research is focus on the preparation of low clay content NC gels containing (less than 6g clay/100ml water). It is found that the mechanical strength of NC
gels increases with increasing hectorite content [11,15,16], but high clay content NC gels are quite difficult to prepare just by simply mixing because of the high viscosity of clay aqueous dispersion at high clay concentration. Recently, using Laponite XLS (Clay-S), a kind of synthetic hectorite modified by tetrasodium pyrophosphate as crosslinkers, we easily solved the difficulty and successfully prepared high clay content Clay-S/poly(N-isopropylacrylamide) NC gels (S-N gels) [17]. S-N gels show excellent mechanical properties, such as high tensile strength,
large elongation, and complicated deswelling behavior, however, their resilience is very poor,limiting their some applications, such as flexible components. In order to get a high resilient NC gels, we successfully prepared a novel NC gel, Clay-S/polyacrylamide NC gel (S-M gel)[18]. The S-M gel show excellent resilience, low hysteresis, and ultrahigh elongation, due to the better hydrophility and flexibility of PAAm molecules. Elongations at break for S-M gels were almost independent on clay content, changing between 2510% and 2830%, much larger than clay/PNIPAAm NC gels(elongation at break: 1000%~1400%) [15,17], while the strength of S-M gels is much smaller than that of S-N gels. Therefore, we think that the mechanical properties of NC gels can be controlled by copolymerization of N-isopropylacrylamide and acrylamide.
In this paper, using N-isopropylacrylamide (NIPAAm) and acrylamide(AAm) as monomers we successfully prepared Clay/poly(N-isopropylacrylamide-co-acrylamide) hydrogels (S-N-M gels), whose mechanical properties can be controlled by adjusting the composition of copolymerization. Furthermore, it is also found that the swelling ratio of S-N-M gels increase with increasing AAm content, but the transparency changes of S-N-M reaction solutions during polymerization decrease with increasing AAm content. We believe that S-N-M gels with controllable mechanical properties and swelling properties will have wide potential applications in mechanical devices, artificial issues etc.
1. EXPERIMENTAL PART
1.1 Materials
Acrylamide (AAm) (98.5%, Shanghai Fine Chemical Material Institute, Chemical Purity),N-isopropylacrylamide (99%, Acros Co., Belgium), synthetic hectorite Laponite XLS (Clay-S)(Rockwood Co., U.S., 92.32wt% Mg5.34Li0.66Si8O20(OH)4 Na0.66, 7.68wt%Na4P2O7), potassium persulfate (KPS), N,N,N’,N’-tetramethyldiamine (TEMED) (Shanghai Chemical Reagent,Analytic Reagent). All reagents were used as received. All solutions used in experiments were prepared in deionized water.
1.2 Preparation of NC Gels
The synthetic procedure of S-N gels and S-M gels is almost the same as the procedures reported previously [17,18]. S-N-M gels were prepared by the same procedure and the total content of monomers were fixed at monomer/water (w/w):1g/10g. In this paper, NC gels are expressed as SxNy, SxMz or SxNyMz where S, M, and N stand for Clay-S, PAAm, and PNIPAAm,respectively, and x, y and z stand for 100×clay/water (w/w), 100×NIPAAm/water (w/w) and 100×AAm/water (w/w), respectively. For example, S15N2.5M7.5 stands for S-N-M gel containing Clay-S with clay/water weight ratio: 15/100, NIPAAm/water ratio: 2.5/100 and PAAm with AAm/water ratio: 7.5/100.
1.3 Measurements of Tensile Properties and Stress Relaxation
Tensile strength measurements were performed on hydrogels of the same size (5mm diameter×60mm length) and the same polymer/water weight ratio (1/10) using a Dejie DXLL-20000. The conditions were as follows: temperature 25℃; a sample size of 5mm in diameter and 60mm length; gauge length of 30mm; crosshead speed of 100mm/min. The strain under stress is defined as the change in length relative to the initial length of the specimen. The strength was calculated on the basis of the initial cross section.
Stress relaxation measurements were performed on hydrogels under the conditions, which were almost the same as tensile strength measurements, except gauge length: 30mm; fixed strains for all gels: 700%, respectively; relaxation time in all measurements: 10 minutes.
1.4 Swelling Behavior
Swelling experiments were performed by immersing as-prepared gels in a large excess of water at room temperature (20℃). For each measurement, the hydrogels were removed from the water and weighted after excess water removed from the surface by wet filter papers. All starting gels were as-prepared hydrogels (prepared at 5℃) with the original water/polymer ratio (w/w)and sample size (5mm diameter×30mm length).
1.4 Swelling Behavior
Swelling experiments were performed by immersing as-prepared gels in a large excess of water at room temperature (20℃). For each measurement, the hydrogels were removed from the water and weighted after excess water removed from the surface by wet filter papers. All starting gels were as-prepared hydrogels (prepared at 5℃) with the original water/polymer ratio (w/w)and sample size (5mm diameter×30mm length).
1.5 Measurement of Transparency during Polymerization
Changes of transparency during polymerization were measured using UV/vis spectrophotometer(UV 1901, Puxi Co. China). The reaction solution was contained in a quartz tube (10mm×10mm×40mm) with a cap, which was regulated at 10℃. Transparency was
recorded continuously during polymerization.
2. RESULTS AND DISCUSSION
2.1 Mechanical Properties of NC Gels
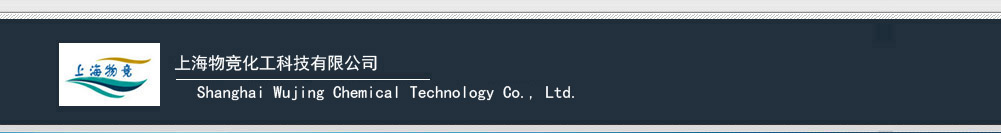
Figure 1 shows effects of different PAAm content on tensile properties of hydrogels. As shown in Figure 1a, all S-N-M gels show high strength, greater than 200kPa, and large elongation, higher than 1000%. As shown in Figure 1b, the tensile strength of S-N-M gels decrease with increasing AAm content, i.e., the strength decreasing from 690kPa for S15N7.5M2.5 to 300kPa for S15N2.5M7.5, while the elongation at break for S-N-M gels increases with increasing AAm content, i.e., the elongation increaseing from 1404% for S15N7.5M2.5 to 1764% for S15N2.5M7.5.
This is a result of the different structure of PAAm molecules and PNIPAAm molecules. The side group of PAAm, –CONH2, is completely hydrated, and the backbone of PAAm is composed of –C–C–, making PAAm excellent flexibility in water. Although the backbone of PNIPAAm is the same as the PAAm, hydrophilicity of side group of PNIPAAm, –CONHCH(CH3)2, is weaker than –CONH2, and the volume of –CONHCH(CH3)2 is larger than that of –CONH2, making PNIPAAm molecules more rigid than PAAm. Thus, the elongation at break of S-M gel is larger than that of clay/PNIPAAm NC gel. As for copolymer, the more NIPAAm units, the more rigid are the copolymer chains; the more AAm units, the more flexible are the chains. Therefore, the tensile strength of S-N-M gels increases with increasing NIPAAm content and their elongation increases with increasing AAm content.
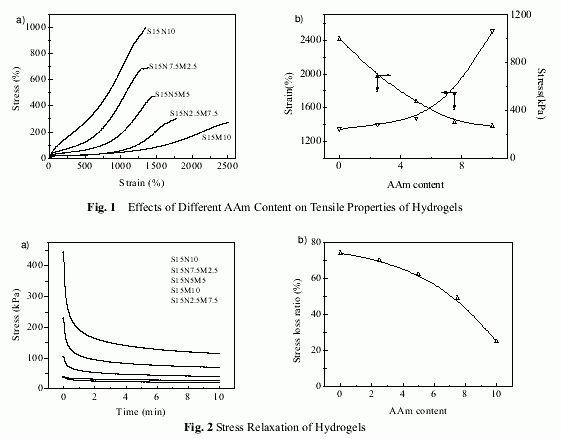
Figure 2 shows stress relaxation of hydrogels. As shown in Figure 2a, stress of S15N10 is lost very quickly with increasing time. (i.e. stress loss is about 49% and 74% during 30s and 10min,respectively.). On the contrary, the stress loss of S15M10 is very small during testing process,about 25% during 10min. The stress loss of S-N-M gels is less than S-N gel, but greater than S-M gel. As shown in Figure 2b, the stress loss of S-N-M gels decreases with increasing AAm content, i.e., stress loss decreasing from 70% for S15N7.5M2.5 to 50% for S15N2.5M7.5.
The remarkable differences of stress relaxation among different hydrogels are also a result of the different size and hydrophilicity of their side groups. On one hand, the larger the side group,the larger the internal friction force. On the other hand, the higher hydrophilicity, the more complete hydration. Then water is a better lubricant in higher hydrophilic polymer networks, and reduces the friction force among molecules to a great extent. As for copolymer, the more PAAm units, the less is the internal friction among copolymer chains. Therefore, the stress loss of copolymer decreases with increasing AAm content.
2.2 Swelling behavior of the NC Gels
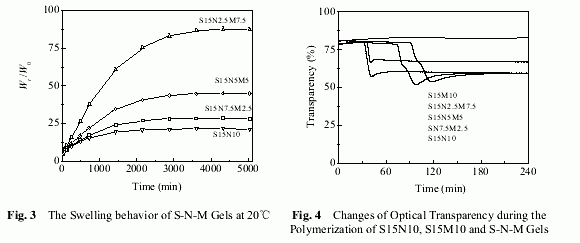
Figure 3 shows swelling behavior of S-N-M gels at 20℃. As shown in Figure 3, swelling ratio of S-N-M gels increases with increasing AAm content. This is a result of the different hydrophilicity of PAAm molecules and PNIPAAm molecules. The copolymer chains containing more AAm units show more hydrophilicity than those containing less AAm units, which leads to higher swelling ratio, i.e., 87g/g for S15N2.5M7.5 much larger than 28g/g for S15N7.5M2.5.
2.3 Transparency Changes during Polymerization
Figure 4 shows changes of transparency during polymerization of NC gels. As shown in Figure 4, optical transparency changes of S-M gels during polymerization are quite different from S-N gels, while the optical transparency changes of S-N-M gels are larger than S-M gel but less than S-N gel. As shown in Figure 4, T% of S15M10 retains 82% during the whole polymerization, no observed obvious change. Transparency of S15N10 reaction solution, which was high (T=80%) at the beginning, abruptly decreases about 74min after starting polymerization. During the decrease of transparency, macroscopic phase separation and precipitation are not observed. After reaching a minimum (T=52%, 98min), transparency increase to a high transparency (T=59%, after 140min). The optical transparency changes of S-N-M reaction solutions during polymerization are very similar to that of S-N reaction solution,except that the minimum transparency is different and increases with increasing AAm content.
Haraguchi reported similar TC phenomenon (transparency changes during polymerization) in NC homopolymer gels[14], prepared using N-substitute acrylamide (NIPAAm,dimethylacrylamide, and diethylacrylamide) or N-acryloylmorphorine as monomers. Haraguchi ascribed transparency changes to the formation of a number of clay/polymer brush particles during the early stages of polymerization, causing the reflection of visible light due to a change in density locally and aggregates of such particles over several tens of nanometers scattering light. However, we found that optical transparency of S15M10 reaction solution are not observed obvious change, (T% retains 82% during polymerization), shown in Figure 4. Meanwhile, the minimum transparency of S-N-M gels increase with increasing AAm contents, i.e., increasing from 54% for S15N7.5M2.5 to 67% for S15N2.5M7.5. According to these results, we conclude that TC phenomenon of NC gels is probably dependent on not only the formation of clay/polymer brush particles but also the hydrophility of polymers. On one hand, a number of clay/polymer brush particles forms during initial stages of polymerization, making a trend to change transparency of reaction system. On the other hand, if the hydrophility of polymer is strong enough to attain good compatibility between clay/polymer brush particles and water (i.e.complete hydration), not causing obvious local density changes, like S15M10, transparency will sustain at the level of initial reaction solution instead of changing. But if the hydrophility of polymer is not strong enough to attain good compatibility between clay/polymer brush particles and water (i.e. incomplete hydration), causing large local density changes, like poly(N-isopropylacrylamide) and poly(N-isopropylacrylamide-co-acrylamide), transparency will change and the minimum transparency during polymerization depend on the hydrophilicity of polymer. For S-N-M gels, the hydrophility of copolymer increases with increasing AAm content,thus the minimum transparency during polymerization become larger.
3. CONCLUSION
In this paper, using N-isopropylacrylamide(NIPAAm) and acrylamide(AAm) as monomers A series of clay/poly(N-isopropylacrylamide-co-acrylamide) nanocomposite hydrogels (S-N-M gels) have been successfully prepared by in situ polymerization. The mechanical properties,swelling behavior of S-N-M gels and the transparency changes can be controlled by adjusting the composition of copolymerization. Compared to traditional hydrogels, S-N-M gels show excellent tensile properties and their swelling ratio increases with increasing acrylamide (AAm) content. The results of stress relaxation indicate that the stress loss decreases with increasing AAm content. It was surprisingly found that the transparency during all S-N-M gel synthesis changes abruptly, and the changes become more abrupt with increasing N-isopropylacrylamide content. The weaker the hydrophilicity of copolymer, the more apparent the transparency change during S-N-M gels polymerization. The relationship between hydrophilicity of copolymer and transparency changes will help to design novel nanocomposite hydrogels. We trust that S-N-M gels with controllable mechanical properties and swelling properties will have wide potential applications in mechanical devices, artificial issues etc.
Acknowledgement
This research is financially supported by the Cultivation Fund of the Key Scientific and Technical Innovation Project, Ministry of Education of China(No.704021), the National Natural Science Foundation of China (Project No.50473002), the National High-tech 863 Project (2002AA302616), and the Shanghai Nano Special Projects (0452nm006, 05nm05005).
|