Wen WU1, Wen Jing LI2, Li Qun WANG1?, Ke Hua TU1, Wei Lin SUN1
1 Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027
2Ningbo Institute of Technology, Zhejiang University, Ningbo 315100
In recent years IPN hydrogels have been attracted attentions as biomedical materials1-3.Among which poly(N-isopropylacrylamide) (PNIPAAm) was frequently used to impart temperature responsive function4, . Many of the second components were introduced forming IPN with PNIPAAm to overcome the drawbacks of poor mechanical property
and lack of sustained release capability of PNIPAAm6.
In this paper, silk sericin (SS) was employed to construct IPN hydrogels with PNIPAAm by a simultaneous polymerization method. SS is one of the natural macromolecular proteins derived from silkworm. Its amino acid composition and unique properties make it an excellent biomaterial and candidate for controlled release of
proteins7. Combining the difference of the solubility of SS in and out of its isoelectric point with the thermo-sensitive property of PNIPAAm, the resulting IPN hydrogels of SS/PNIPAAm showed temperature-pH sensitive property.
SS/PNIPAAm hydrogels were prepared by the simultaneous-IPN method. SS and NIPAAm monomer were mixed in predetermined feed ratios in deionized water with the total polymer concentration of 20wt%. The mixtures were stirred for 30 minutes under nitrogen atmosphere. Then the crosslinkers, glutaraldehyde (GA) (5wt% of SS) and N,N′-methylenebisacrylamide (MBAAm) (3 mol% of NIPAAm) and the initiators ofN,N,N′,N′-tetramethylethylenediamine(TMEDA)(1wt% of NIPAAm) and ammonium
peroxydisulfate (APS) (1wt% of NIPAAm) were added. After vigorous stirring for about1 minute, the mixture was poured into a sealed glass mold and reacted at 25±0.2℃ for 3 days under nitrogen atmosphere. The resulted IPN plates were immersed in deionizedwater at room temperature for five days during which the water bath was refreshed every 12 hrs to remove reagent residues completely. The IPN hydrogels was then cut into small discs (15 mm in diameter and 3 mm in thickness) and thoroughly dried under vacuum. The IPN hydrogels were donated as IPNxy where x and y present the feed contents of SS and NIPAAm, respectively. The homo-PNIPA-Am and SS hydrogels were prepared with the same procedure, and donated as PNIPAAm and SS hereafter.The amounts of NIPAAm and SS leached out after gel formation are presented in Table1.
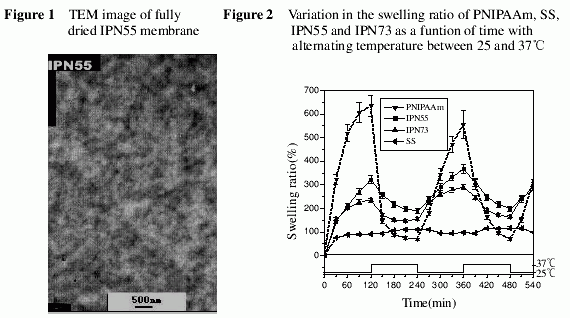
The morphology of the IPNs was investigated by transmission electron microscopy (TEM) performed on JEOL JEM-1230 EX. The SS phase in the IPNs were stained by immersing the IPN samples in 0.1 mol/L ammonium cuprate solution for 12 hrs, wherein coordinate reaction between SS and copper (II) occurred forming SS-Cu(II) complex8.
The stained samples were purified by immersed in deionized water for 3 days with refreshing the water bath every 12 hrs to remove the residues. The dried samples were cut into thin films with ultra-microtome.
Stepwise swelling behaviors were studied by gravimetric method in deionized water with temperature alternating between 25 and 37℃. The pH values of the buffer solutions were changed from 2 to 11. The swelling ratio of the IPN hydrogels was determined according to the following equation:
Swelling ratio (%) = [ (Ws-Wd) / Wd ]×100
Where Ws and Wd are the weight of swollen and dry samples, respectively.

The morphology of the IPN55, shown in Figure 1, demonstrates that there was no clear boundary between the two phases, and the domain sizes in a relatively micro scale.The result suggested that the compatibility of SS and PNIPAAm are in the some extent.
Figure 2 shows the variation of swelling ratios with temperature for PNIPAAm, SS,IPN55 and IPN73. The swelling behavior of SS was little affected by the temperature change. Its swelling ratio increased from 90% to about 110% when the temperature was elevated from 25℃ to 37℃. In contrast, both of the IPN55 and IPN73 exhibited relatively sharp temperature-dependent swelling profiles, though the covering range was less than that of PNIPAAm. At 25℃ the swelling ratio reached more than 320% for IPN55 and 230% for IPN73. When the temperature was elevated to 37℃, the swelling ratios of the IPNs dropped abruptly, showing a significantly temperature dependence.These swelling processes are reversible and repeatable with temperature.
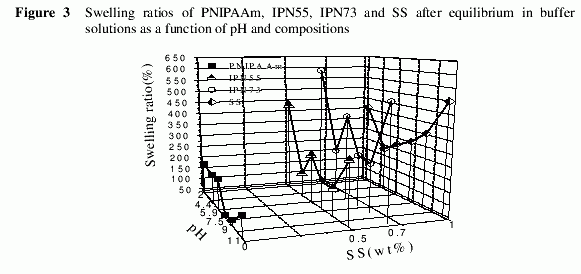
Dependence of the swelling ratios on pH and the composition for PNIPAAm, IPN55,IPN73 and SS at 37℃ is presented in Figure 3. The swelling ratio for PNIPAAm ranged at about 130% when the pH is below 7. When the pH value was higher than 7.5,PNIPAAm showed lower swelling ratio due to the increase of ionic strength in the buffer solution, which was recognized as salting-out effect9. For SS, since its isoelectric value fell in pH 4.4, the swelling ratio of which reached the minimum. Increasing or decreasing the pH values of the buffer solution resulted in the increase of the swelling ratio of SS. It is seen that the swelling ratios of IPN55 and IPN73 were very sensitive to pH. For IPN55 the swelling ratio reached to the minimum of 140% at pH 4.4.When the pH increased to 5.9, the swelling ratio reached as high as 245%.
The same tendency was exhibited in the swelling profile of IPN73, where the lowest swelling ratio reached to 237% at pH 4.4 and increased to 404% at pH 5.9. It was also found that the balanced swelling ratio was significantly affected by the composition of the IPNs, suggesting that the swelling profile of the IPN can be readily modulated by changing the feed ratio in the course of preparing the IPN. Therefore, the IPNs composed of SS and PNIPAAm can be used as pH and temperature-response of hydrogels.
|