Responsive Properties
Shao Hua SHI1,2,*, Li Jian LIU1*
1Department of Polymer Science, College of Chemistry and Molecular Science, Wuhan University
Key Laboratory of Biomedical Polymers (Wuhan University), Ministry of Education,
Wuhan 430072
2R & D Center, Nhwa Pharmaceutical Group, Xuzhou 221007
Poly(N-isopropylacrylamide) (PNIPAM) hydrogel, which is insoluble but shrinkable or
swellable in aqueous media when temperature rises or drops across 33oC1,2, has been extensively studied due to its potential applications in the fields of controlled drug release3, enzyme immobilization4, tissue engineering5 and bioseparasion6. For these applications the rates of swelling and shrinking of the PNIPAM hydrogel were of significant importance, therefore a variety of approaches were explored to accelerate the responsive rates of PNIPAM hydrogels, including: (i) tailoring PNIPAM molecular architecture by grafting hydrophilic side groups such as poly(ethylene oxide)7, carboxy group8 and poly(N-isopropylacrylamide) oligomer groups9. (ii) using pore forming agents, for instance, polyethylene glycol10, CO2 bubbles11 and CaCO3 . (iii) changing polymerization conditions, e.g., conducting polymerization above the lower critical
solution temperature (LCST)1,2, under freezing condition13 and in sucrose solution14 etc..
Due to the homogeneity, high efficiency and low energy consumption of microwave heating, microwave-assisted chemical synthesis has attracted wide attention15, and microwave-assisted heating could accelerate the rates of polymerization reaction16 and improve the properties of the products. In this communication, we report microwave-assisted preparation of PNIPAM hydrogel with improved responsive properties such as faster swelling and shrinking rates.
Preparation of PNIPAM hydrogels A microwave oven with working frequency at 2.45 GHz was used in our experiments.
800 mg N-isopropylacrylamide, 5 mg N, N′-azobisisobutyronitrile and 50 mg N, N′-
methylenebisacrylamide were placed into a glass ampoule, and 3 mL 1:1 (v/v) ethanol/water was added. The ampoule was induced N2 and degassed for three times and sealed.
The reaction mixture was irradiated under microwave until the temperature in the
reaction mixture reached 56oC. The hydrogel thus prepared was immersed in water,and was refreshed with fresh water every few hours to remove unreacted chemicals anddried at 40oC in vacuum. The thermal polymerization was carried out at 62oC for 10min. The hydrogel prepared under microwave irradiation and by thermal heating were designated as MH and TH.
It was found that the polymerization/crosslingking reaction of MH under 75 W
microwave irradiation completed in 40 s with a yield of 99 %, whereas the reaction of TH at 62 C needed 5 min to form a bulk hydrogel with approximately the same yield.Kinetics of swelling and shrinking The kinetics of swelling and shrinking were gravimetrically measured. The dry gel disc or the gel sample swollen to equilibrium at 25oC was transferred into 25oC or 37oC water for swelling or shrinking. At regular time intervals, the changes of the hydrogel weights were recorded after blotting the excess surface water with moistened filter paper
until constant weights were reached. Water ratio (WR) is defined according to the
following equation:
WR (%)=100 x (Wt-Wd)/(W25-Wd)
Wt is sample weight at time t, Wd is weight of dry sample and W25 is weight of sample
equilibrated in 25℃.
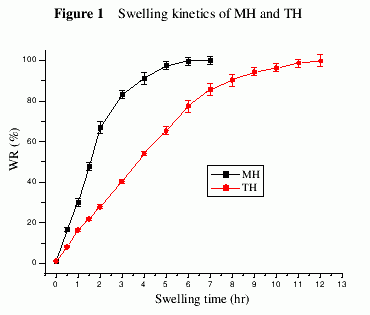
As shown in Figure 1, MH swelled faster than TH. After 2 hr MH absorbed about
67% water, while TH only absorbed 27% water. On the other hand MH only took
about 6 hr to reach equilibrium at 25oC, whereas TH needed 13 hr.
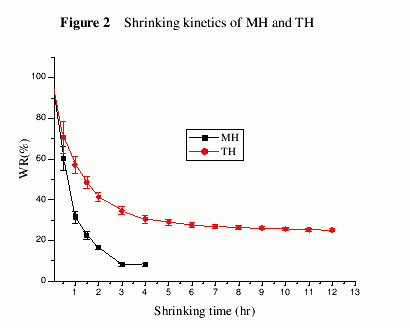
It was seen from Figure 2 that the shrinking rate of MH was tremendously greater than that of TH. MH lost about 68% of water within 1 hr, however, TH only lost 36.4% of water. MH only needed 3 hr for reaching equilibrium, while TH reached the deswelling equilibrium after 12 hr.
The improved responsive properties of MH were attributed to its more
heterogeneous and porous structure. It has been reported that the fast responsive PNIPAM hydrogels with heterogeneous and porous networks were prepared by polymerization above LCST due to microphase separation1,2. Under microwave irradiation the temperature of the reaction system rose rapidly, further accelerated the rate of phase separation for PNIPAM network and formed a more heterogeneous structure.In addition, the interior heating of microwave acted as an exerting pressure upon the hydrogel network to make it to expand outward and form macroporous networks.
Acknowledgement The research was financially supported by the National Natural Science Foundation of China (No.20274032), the Ministry of Education; the 973 Project of China (G1999064703) and Nhwa
Pharmaceutical Corporation.
References
1. B. G. Kabra, S. H. Gehrke, Polym. Commun., 1991, 32(11), 322.
2. X. S. Wu, A. S. Hoffman, P. Yager, J. Polym. Sci., Part A: Polym. Chem., 1992, 30(10), 2121.
3. C. F. Lee, C. J. Wen, C. L. Lin, W. Y. Chiu, J. Polym. Sci., Part A: Polym. Chem., 2004,
42(12), 3029.
4. C. D. Liang, A. S. Hoffman, ACS Sympos. Series, 1987, 350, 236.
|