XU YaXin, LI HongTu, ZHANG Kai, ZHANG Bao, AI Peng & WANG JingYuan
Alan G. MacDiarmid Institute of Jilin University, Changchun 130012, China
“Living” polymerization of vinyl monomers is a route to
synthesize a wide range of well-defined polymers. It
allows the synthesis of polymers with well-defined com-
positions, architectures, functionalities, chain topology
of macromolecules with narrow molecular weight dis-
tribution, targeted number-average molecular weights,
specific end groups, etc.[1]. ATRP fulfils these require-
ments by using a transition metal in combination with a
suitable ligand[2,3]. ATRP is a versatile technique for the
controlled polymerization of wide variety of functional
monomers and has been used successfully to prepare
well-defined polymers, such as styrene, substituted sty-
renes, (meth)-acrylates, and acrylonitrile. In ATRP, the
development of new/functionalized initiators permits to
prepare a wide range of new materials with improved
properties, which are either difficult to prepare or not
available via other polymerization techniques.
Nowadays, there is a considerable interest not only in
the synthesis of new types of polymeric materials, but
also in the modification of existing polymers in order to
alter their properties to meet requirements for new ap-
plications. One of the methods for modification is the
grafting reaction, which provides an opportunity to vary
physical and chemical properties of polymers. Graft co-
polymers can be obtained with three general met-
hods[4―7]: (i) grafting-onto, in which side chains are
preformed and then attached to the backbone; (ii) graft-
ing-from, in which the monomer is grafted from the
backbone; (iii) grafting-through, in which the macro-
monomers are copolymerized. The graft copolymers
obtained by ATRP allows the control of the length and
the composition of both the backbone and the side
chains.
Poly(N-isopropylacrylamide) is a thermoresponsive
polymer which is water soluble at room temperature and
is able to give a coil-to-globule transition above 32.8℃
(the lower critical solution temperature, LCST)[8]. The
block copolymers and graft copolymers based PNIPAM
have attracted much attention as the LCST in water is
close to body temperature and may, therefore, be applied
in the biomedical field as a stimulus-sensitive materi
als[9―11].
In this paper, we report a successful ATRP of NIPAM
using the brominated polystyrene as initiator and CuCl
combined with HMTETA as catalyst in the N,N-di- me-
thylformamide(DMF) system. The resulting copolymers
were determined by means of H NMR and GPC. The
thermal property of PSt-g-PNIPAM was analyzed by
DSC.
1 Experimental
1.1 Materials
Styrene was first washed with an aqueous solution of
sodium hydroxide (5 wt %) three times and with water
until neutralization and then distilled under reduced
pressure. N-isopropylacrylamide (NIPAM, Aldrich) was
purified by recrystallization from acetone. 4,4′-Azobis
(isobutyronitrile) (AIBN, Fluka, 98%) were purified by
recrystallization from ethanol. Tetrahydrofuran (THF)
was distilled from a purple sodium ketyl solution. CuCl
purchased from Shanghai Chemical Co. (A.R., 97.0%)
was purified by stirring in acetic acid, filtered, washed
with ethanol and dried. DMF was distilled before use.
Ethyl 2-bromoisobutyrate (EBrIB), 2,2′-bipyridine (Bei-
jing chemical Co.), Hexamethyltriethylenetetramine
(HMTETA, Aldrich), CCl4(99%) and N-bromosuccin-
imide (NBS) were used as received without further puri-
fication.
1.2 Synthesis and bromination of PSt
The procedures for synthesis of PSt by ATRP can be
found elsewhere in detail[12]. H NMR (CDCl3), δ:
2.21―1.29 (CH2CH) and 7.32―6.30 (aromatic protons)
(Figure 1(a)). Mn: 10235 g/mol; Mw/Mn: 1.21.
In a typical experiment, NBS (0.342 g, 1.92 mmol)
and AIBN (0.052 g, 0.32 mmol) were added to a sus-
pension of polystyrene (1.5 g, styrene: 2.74% (molar
content)) in CCl4 (60 mL) in a 100 mL round-bottomed
flask with a magnetic stirring bar. After refluxing at
90℃ for 6 h, the reaction mixture was filtered, the fil-
trate was in large part removed by rotary evaporation
and precipitated in methanol, and then the product was
dried overnight at 30℃ under vacuum. Mn: 10980 g/mol;
Mw/Mn: 1.24. The bromine content of the product, de-
termined through elementary analysis, was 9.94% (mo-
lar content). H NMR (CDCl3), δ : 2.21―1.29 (CH2CH),
3.95 (CH2CBr) and 7.32―6.30 (aromatic protons) (Fig-
ure 1(b)).

1.3 Synthesis of PSt-g-PNIPAM
In a typical experiment, a dry round-bottomed flask was
charged with DMF (3 mL), CuCl (0.043 g, 0.3 mmol),
HMTETA (0.051 g, 0.3 mmol), NIPAM (0.910 g, 8
mmol), and PSt-Br (0.25 g, 0.23 mmol of Br). The flask
was sealed, and O2 was removed by three cycles of
freeze-pump-thaw. Then the flask was filled with puri-
fied Ar. After the mixture was allowed to stir at ambient
temperature for 5 min, the flask was placed in an oil
bath at 90℃ for 24 h. The reaction mixture was im-
mersed in water for 24 h in order to remove PNIPAM
homopolymer and unreacted monomer. The crude prod-
uct was dissolved in DMF and precipitated into petro-
leum ether (b.p. 30―60℃); precipitation was repeated
three times. The graft copolymer was then filtrated and
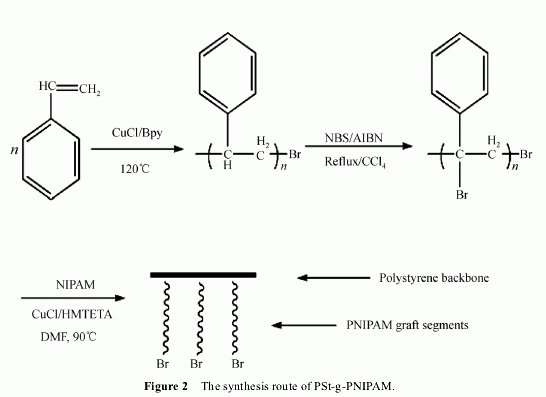
dried at 40℃ in a vacumm oven for 24 h. Figure 2
shows the synthesis route to the graft copolymer. Mn:
19815 g/mol; Mw/Mn: 1.35. H NMR (CDCl3), δ: 5.81―
7.05(NHCH(CH3)2), 4.04[NHCH(CH3)2], 2.42 ― 1.29
(CH2CHCO), 1.20(NHCH(CH3)2), 2.21―1.29 (CH2CH)
and 7.32―6.30 (aromatic protons).
1.4 Characterization
IR Spectra of KBr powder-pressed pellets were recorded
on a BRUKER VECTOR22 Spectrometer. Elemental
analysis results were obtained on a Flash Ea 1112 ele-
mental analysis instrument. Measurements of nuclear
magnetic resonance (NMR) spectra were conducted on a
Broker ARX-500 NMR spectrometer with CDC13 as
solvent, using trimethylsilane (TMS) as internal standard.
Molecular weights and molecular weight distributions
were measured on a Waters 410 Gel permeation chro-
matography (GPC) apparatus equipped with a 10-μm
Styragel HT6E column (300 mm×7.8 mm) using linear
polystyrene standards. DMF was used as the eluent at a
flow rate of 1 mL/min. The glass transition temperature
(Tg) of the graft copolymer was conducted by a
METTLER TOLEDO DSC 821e instrument under liq-
uid nitrogen. The sample was heated from 25 to 400℃
at a heating rate of 20℃/min.
2 Results and discussion
2.1 Bromination reaction
Bromination was carried out using a suspension of PSt,
in CCl4 in the presence of NBS and AIBN (Figure 2).
The H NMR spectra of the starting PSt (Figure 1(a))
reveal the presence of signals characteristic of PSt at δ
(TMS) 2.21―1.29(CH2CH) and 7.32―6.30 (aromatic
protons). After bromination, the intensity of the CH
resonance (δ 1.9) decreased significantly due to benzylic
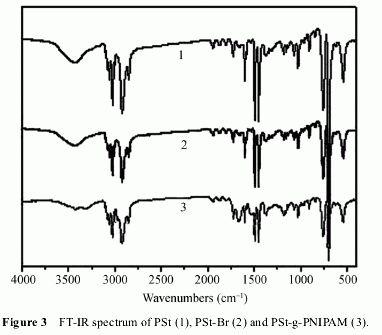
bromination (Figure 1(b))[13]. In the IR spectra (Figure
3), the adsorption peaks at 2960, 1600, 1454 cm?1,
which are the characteristic peak present in PSt. Com-
pared to PSt, the IR spectra PSt-Br has no obvious
change. The bromine content of this product, as deter-
mined through elementary analysis, was 9.94 % (molar
content).
2.2 Grafting reaction
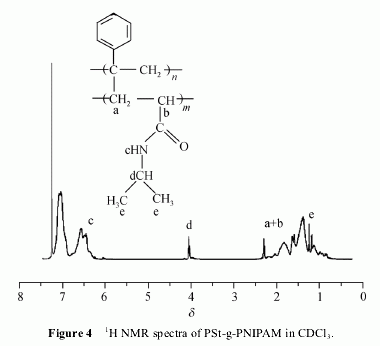
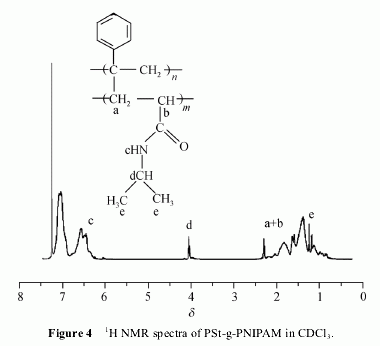
The H NMR spectrum in CDCl3 of the final product
(Figure 4) reveals the presence of signals from both PSt
and PNIPAM block. Characteristic signals from
PNIPAM at δ 5.81―7.05 (NHCH(CH3)2), 4.04 (NHCH
(CH3)2), 2.42―1.29 (CH2CHCO), 1.20 (NHCH(CH3)2)
ppm and the presence of signals from PSt at δ 2.21―
1.29 (CH2CH) and 7.32―6.30 (aromatic protons) were
obtained.
Figure 3 is the IR analysis of the PSt(1), PSt-Br(2),
and PSt-g-PNIPAM(3). In the IR spectra of PSt-g-
PNIPAM, there is a new adsorption peak at 1675 cm-11,
which is a characteristic peak of the C=O. The adsorp-
tion peaks at 1541 and 3300 cm?1 result from N―H
stretching.
The starting PSt(1), PSt-Br(2), and the final graft co-
polymer, PSt-g-PNIPAM(3), are characterized by GPC
(Figure 5) in DMF using polystyrene calibration. Three
narrow distributed polymers are obtained: Mn=10235
g/mol, Mw/Mn =1.21, Mn =10980 g/mol, Mw/Mn =1.24,
and Mn = 19815 g/mol, Mw/Mn = 1.35, respectively. The
polydispersity index (PDI) was lower than 1.5, as ex-
pected for a “living” process. The GPC traces in Figure
5 were reasonably symmetrical, with no shoulders, and
the decrease in the elution time was consistent with the
increase in the molecular weight, which revealed a new
well-defined PNIPAM block was grafted from the
PSt-Br macroinitiator by ATRP, and the GPC trace pro-
vides no evidence of the homopolymer PNIPAM and
PSt-Br in the graft copolymer.
The Mn and PDI of the PSt, PSt-Br, and PSt-g-
PNIPAM, deduced from both GPC and H NMR results,
are listed in Table 1. The Mn of 28300 g/mol for the PSt-
g-PNIPAM, deduced from H NMR results is much
higher than the corresponding Mn of 19815 g/mol, de-
duced from GPC results. The deviations were probably
caused by the fact that the molecular weight of the co-
polymer was calculated based on PS standards using a
refractive index detector; they were lower than their true
values because branched polymers have smaller hydro-
dynamic volumes than corresponding linear counterparts
with the same molecular weights.

On the other hand, because the graft polymerization
system with PSt-Br macroinitiator is viscous, the effi-
ciency of macroinitiator is low. The polymerization
needs high temperature and long time, and is incom-
plete.
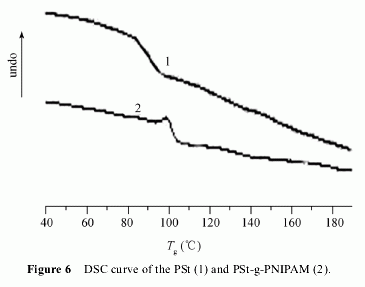
The thermal properties of PSt-g-PNIPAM was ana-
lyzed by DSC (Figure 6). The starting PSt had a glass
transition temperature (Tg) at about 84.1℃. It was lower
than the normal one at about 100℃ because of the com-
parative low molecular weight of PSt (Mn = 10235
g/mol). Low molecular weight polymers exhibit lower
Tg. The Tg value for our graft copolymer is 100.1℃
which is higher than that of the starting polymer. Due to
the carbonyl group derived from PNIPAM was intro-
duced into PSt, electronegativity of the carbonyl group
is so intense that intrachain and interchain hydrogen
band easily form, and H bonding contributes 25―41
kJ/mol toward molecular stability and affect the cohe-
sion energy density, which influences Tg. Furthermore,
molecular asymmetry of the chemical structure arises by
the incorporation of isopropyl with bulky volume de-
rived from PNIPAM. Isopropyl makes it inflexible that
C―C band rotates and increases Tg.
3 Conclusion
We present a successful example of structurally well-
defined, polystyrene-based graft copolymers through
atom transfer radical polymerization. The H NMR
spectrum and the unimodal and symmetrical shape of
the trace obtained at GPC proved that the graft copoly-
mer were obtained. Due to the fact that the carbonyl
group derived from PNIPAM was introduced into PSt,
the Tg value for the graft copolymer is higher than that
of the starting PSt. On the side, experiments aiming at
the thermoresponsive properties of PSt-g-PNIPAM are
currently under way.
|